5 AI Tools for Drug Safety Monitoring
AI is changing the way drugs are monitored for safety by providing faster, more accurate insights into adverse drug reactions (ADRs). Traditional methods often miss over 90% of ADRs, but AI tools now analyze massive datasets like electronic health records, social media, and insurance claims to detect issues earlier. These tools also automate time-consuming tasks like case processing and regulatory submissions, saving resources and improving compliance. Below are five AI tools reshaping drug safety monitoring:
- Deep Intelligent Pharma: Automates case processing, extracts data from unstructured sources, and ensures regulatory compliance.
- Oracle: Offers advanced statistical analysis and automation for large-scale safety operations.
- Veeva Systems: Provides an all-in-one cloud platform for streamlined drug safety processes.
- ArisGlobal: Uses AI to reduce false positives and speed up evaluations.
- IQVIA: Excels in analyzing social media and unstructured data for real-time safety insights.
These tools cater to different needs, from handling high case volumes to ensuring real-time monitoring, making them essential for improving patient safety while meeting regulatory demands.
Advance Drug Safety With Oracle’s AI-Powered Pharmacovigilance
1. Deep Intelligent Pharma
Deep Intelligent Pharma stands out as a powerful AI tool for drug safety monitoring, simplifying complex workflows while improving both signal detection and adherence to regulatory standards.
Automation of Pharmacovigilance Workflows
One of the most labor-intensive aspects of pharmacovigilance is processing Individual Case Safety Reports (ICSRs). Deep Intelligent Pharma addresses this challenge by using AI to handle tasks like data intake, validation, and coding. It extracts vital information from unstructured sources such as medical narratives and lab reports, effectively eliminating the need for manual data entry. With tools like OCR and NLP, the platform digitizes documents and pulls critical safety data from text-heavy formats. For example, when physician notes or lab results are submitted, the system extracts key safety details, significantly cutting down processing delays. This automation supports quicker and more efficient safety monitoring.
Signal Detection and Adverse Event Reporting
The platform’s ability to process data quickly feeds into its advanced analytics for safety monitoring. Deep Intelligent Pharma uses a multi-agent system architecture, where multiple AI agents collaborate to enhance detection capabilities. For instance, one agent might monitor real-time data streams, another cross-checks against known drug effects, and a third combines these insights to identify new safety signals. This layered approach uncovers emerging patterns that might go unnoticed with traditional methods.
In addition to detecting signals, the platform ensures that adverse event reporting aligns with stringent regulatory requirements.
Compliance with Regulatory Standards
Adhering to FDA regulations, such as 21 CFR 314.80 and 21 CFR 600.80, is a critical component of drug safety monitoring in the U.S.. Deep Intelligent Pharma simplifies this by automatically mapping adverse events and medications to standardized dictionaries like MedDRA and WHODrug. This ensures that regulatory submissions are consistent and meet the uniformity standards expected by U.S. authorities, reducing the chances of delays or rejections during the approval process.
2. Oracle
Oracle is transforming post-market drug surveillance by integrating automated workflows with advanced statistical analysis through its Safety One Intake and Empirica Signal platforms.
Automation of Pharmacovigilance Workflows
Oracle's Safety One Intake streamlines the processing of adverse event documents from multiple sources, such as email, APIs, and Electronic Data Interchange (EDI). Using OCR and machine learning, the platform extracts key details, classifies documents, identifies duplicates, and merges follow-up data to update or create safety cases in Oracle Argus Safety. This automation significantly reduces manual effort, as highlighted by Randy Thompson, Chief Health Analytics Officer at Billings Clinic:
"Oracle Clinical AI Agent has allowed us to focus on improving the physicians' lives by reducing the documentation and cognitive burden and allowing physicians to spend more time doing the things they love".
Beyond automation, Oracle strengthens drug safety monitoring with its advanced signal detection capabilities.
Signal Detection and Adverse Event Reporting
Oracle's Empirica Signal takes a data-driven approach to uncover potential safety risks. It calculates statistical scores for product-event combinations across vast safety databases. Using methods like Multi-item Gamma Poisson Shrinker (MGPS), Regression-adjusted Gamma Poisson Shrinker (RGPS), and Proportional Reporting Ratios (PRR), the platform identifies unexpected drug-event associations. Impressively, it can handle combinations involving up to five variables, enabling the analysis of complex drug interactions and syndromes. The system also integrates data from major regulatory databases such as FAERS, VAERS, and VigiBase, allowing users to explore detailed case lists from high-level signal scores.
Compliance with Regulatory Standards
Oracle’s pharmacovigilance tools are built with compliance in mind. They maintain detailed audit trails to document every change in the safety management process, ensuring accountability during inspections. The platform adheres to strict data protection guidelines, including GDPR and HIPAA, to safeguard patient information. Additionally, its AI framework ensures transparency and interpretability of outputs. By consolidating clinical, operational, and financial data into a unified system, Oracle enables data-driven decision-making that aligns with regulatory requirements.
3. Veeva Systems
Veeva Systems simplifies drug safety monitoring by consolidating everything onto a single cloud platform: the Veeva Vault Safety Suite. This eliminates the fragmented data management often seen with older systems. The platform integrates processes like intake, case processing, signal detection, and safety content management seamlessly - no custom interfaces or third-party tools needed. By streamlining these elements, Veeva underscores the importance of end-to-end drug safety monitoring.
Automation of Pharmacovigilance Workflows
Veeva leverages AI to handle routine pharmacovigilance tasks within its cloud-based workflows. For instance, the Case Intake Agent extracts data from source documents and flags potential issues, while the Case Narrative Agent polishes grammar and consolidates information for better readability. Automated processes also transfer clinical SAE data and synchronize product metadata across regulatory and safety vaults, ensuring consistency throughout.
One client was able to deploy Vault Safety in just 12 weeks, gaining real-time oversight and streamlined compliance in record time. Cory Gilbert, Senior Director of PV Operations & Global Process Enablement, highlighted this efficiency:
"The technology becomes a key enabler to being able to work more effectively and enabling our pharmacovigilance colleagues to do the things that they get excited about".
This kind of automation lays a strong foundation for effective signal detection.
Signal Detection and Adverse Event Reporting
The Veeva Safety Signal tool uses PRR and ROR algorithms to identify potential safety concerns. It pulls curated data nightly from sources like FAERS, VAERS, and EudraVigilance, utilizing Amazon Redshift to ensure a consistent and reliable data pipeline. The system prioritizes findings that are statistically significant and manages the signal validation process according to GVP Module IX guidelines.
Compliance with Regulatory Standards
Veeva Safety not only improves operational efficiency but also ensures compliance with regulatory requirements. It supports FDA E2B(R3) standards for ICSRs and includes built-in gateways for direct submissions to the FDA. The platform offers three product updates per year to stay aligned with evolving regulatory demands and ensures full traceability, linking detected signals back to their source safety cases for audits. By 2019, 4 of the top 10 pharmaceutical companies by revenue had adopted Veeva Vault RIM to enhance their regulatory operations.
sbb-itb-58f115e
4. ArisGlobal
ArisGlobal's LifeSphere MultiVigilance is recognized as the first end-to-end safety system in the industry with production-ready automation. By leveraging natural language processing (NLP) and machine learning, the platform automates critical tasks like case management, duplicate checks, coding, and triage. This allows safety teams to shift their focus from repetitive data entry to more strategic decision-making. Below, we explore how the platform's modules improve efficiency, accuracy, and compliance.
Automation of Pharmacovigilance Workflows
The LifeSphere NavaX engine utilizes advanced AI to streamline repetitive safety processes. Its Advanced Intake module, powered by GenAI, dynamically extracts safety data from medical narratives and lab reports, cutting case intake times by up to 65%. Additionally, the MedDRA Coding Agent, which received recognition from Frost & Sullivan in 2025, automates complex coding workflows with minimal human input. This not only enhances coding consistency but also significantly reduces processing time.
Beyond these efficiencies, the platform's signal detection capabilities further strengthen safety monitoring efforts.
Signal Detection and Adverse Event Reporting
ArisGlobal's Advanced Signals tool employs AI-driven analytics to identify high-quality safety signals while filtering out irrelevant data. This enables up to 80% faster case assessment during signal detection and reduces false positives by 40–50% compared to traditional methods. The LifeSphere Reporter portal integrates seamlessly with safety and medical systems, allowing stakeholders to quickly access and report field cases - speeding up field case reporting by as much as 50%. Additionally, literature management benefits from AI-powered reference processing, which operates 70% faster than manual approaches.
Compliance with Regulatory Standards
The platform's cloud-native SaaS design ensures that 100% of LifeSphere Safety customers remain compliant with current and future regulatory requirements. Regular cloud updates automatically align the system with evolving standards such as ICH E2B(R3) and ISO IDMP. Automated distribution rule management handles updates and new product additions, reducing manual compliance checks by 70%. ArisGlobal also collaborates with key regulatory bodies like the FDA, Health Canada, and NMPA, ensuring its technology meets official expectations. This alignment supports over 220 global life sciences companies.
5. IQVIA
The IQVIA Vigilance Platform is a SaaS solution that brings AI into every phase of pharmacovigilance. It’s built to handle vast amounts of safety data while ensuring regulatory compliance and lightening the administrative load for safety teams.
Automation of Pharmacovigilance Workflows
The platform’s Vigilance Intake module automates tasks like data ingestion, classification, and extraction from various sources, such as emails, E2B files, and structured forms. For example, in August 2024, Sanofi partnered with IQVIA to launch the third phase of its ARTEMIS project (Adverse Event Processing using Technology-Enabled Medical and Intelligence Solutions). Within less than a year, Sanofi transitioned to IQVIA’s system, aiming to fully replace its legacy processes by 2025. This shift has already eased the manual workload for over 700,000 adverse reaction reports annually. As Daunielle Chipman, Senior Director at IQVIA, explains:
"The primary goal is to refocus safety teams on high-value activities, such as medical review, by alleviating the heavy manual administrative burden they currently face".
Signal Detection and Adverse Event Reporting
IQVIA’s platform takes safety a step further with Vigilance Detect, which uses AI, Natural Language Processing (NLP), and Optical Character Recognition (OCR) to identify adverse events (AEs) and product quality complaints (PQCs). It scans unstructured data sources like social media, audio files, and patient support programs. For instance, the system monitors 2.6 million social media records from 300 sources across 31 countries. During a 30-day analysis, it reviewed 850,000 cases across 91 products, achieving 100% accuracy in detecting missed pregnancy reports. In another instance, it analyzed 23,000 call notes and identified 109 AEs with perfect accuracy. For a patient support program managing 1.2 million records annually, Vigilance Detect reduced manual review costs by 50%.
Compliance with Regulatory Standards
In addition to its automation and detection capabilities, IQVIA emphasizes strict regulatory compliance. Its tools are GxP (CFR Part 11) validated and aligned with the FDA’s January 2025 AI guidance. The platform also participates in the FDA’s Emerging Drug Safety Technology Program (EDSTP), which offers feedback on AI use in pharmacovigilance systems. Archana Hegde, Senior Director of Integrated PV Solutions at IQVIA, explains:
"The FDA's guidance centers on a risk-based credibility model. This means the level of scrutiny applied to an AI system depends on how influential it is in regulatory decision-making".
IQVIA’s system-agnostic design ensures smooth integration with existing workflows, allowing organizations to modernize their safety operations without compromising compliance. This blend of automation and regulatory alignment highlights IQVIA’s role in advancing drug safety monitoring.
Feature Comparison
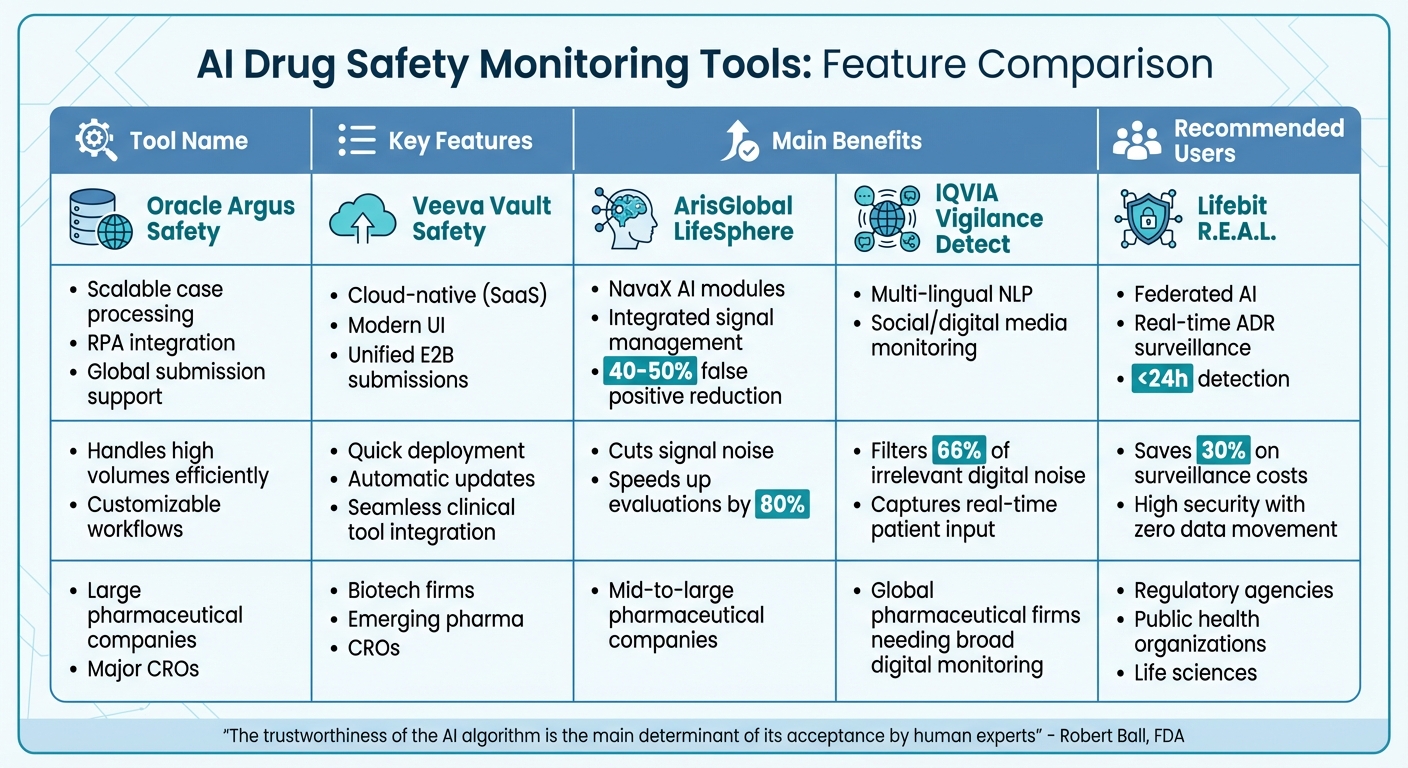
AI Drug Safety Monitoring Tools Comparison: Features, Benefits, and Best Users
This section provides a quick overview of the standout features and benefits of five AI tools designed for post-market drug safety monitoring. Each tool caters to different operational scales and regulatory priorities, offering unique strengths. The table below summarizes their key offerings, benefits, and ideal users.
| Tool Name | Key Features | Main Benefits | Recommended Users |
|---|---|---|---|
| Oracle Argus Safety | Scalable case processing, RPA integration, global submission support | Handles high volumes efficiently; customizable workflows | Large pharmaceutical companies and major CROs |
| Veeva Vault Safety | Cloud-native (SaaS), modern UI, unified E2B submissions | Quick deployment; automatic updates; integrates seamlessly with clinical tools | Biotech firms, emerging pharma, and CROs |
| ArisGlobal LifeSphere | NavaX AI modules, integrated signal management, 40–50% false positive reduction | Cuts signal noise; speeds up evaluations by 80% | Mid-to-large pharmaceutical companies |
| IQVIA Vigilance Detect | Multi-lingual NLP, social/digital media monitoring | Filters 66% of irrelevant digital noise; captures real-time patient input | Global pharmaceutical firms needing broad digital monitoring |
| Lifebit R.E.A.L. | Federated AI, real-time ADR surveillance, <24h detection | Saves 30% on surveillance costs; ensures high security with zero data movement | Regulatory agencies, public health organizations, and life sciences |
This table highlights each tool's core strengths and their best-suited users. For instance, Oracle Argus Safety is tailored for enterprises managing large-scale case volumes, while Veeva Vault Safety offers a modern, cloud-based solution that’s perfect for smaller, agile organizations. ArisGlobal LifeSphere simplifies safety evaluations by reducing false positives, making it a great fit for mid-to-large pharmaceutical companies. IQVIA Vigilance Detect shines in real-time social media monitoring, filtering out irrelevant chatter to focus on genuine safety concerns. Finally, Lifebit R.E.A.L. stands apart with its federated AI approach, ideal for public health bodies and regulatory agencies prioritizing security and real-time insights.
These tools significantly lighten the workload in case processing and surveillance. As Robert Ball, MPH, ScM, MD, aptly states:
"The trustworthiness of the AI algorithm is the main determinant of its acceptance by human experts"
This underscores the importance of maintaining human oversight to ensure these systems meet quality and compliance standards.
Ultimately, selecting the right tool hinges on your organization’s specific goals - whether it's managing high case volumes, leveraging cloud-native flexibility, or ensuring advanced real-time monitoring. Each tool offers tailored solutions to meet distinct operational and regulatory demands.
Conclusion
AI plays a pivotal role in drug safety monitoring, addressing challenges that traditional methods simply can't scale to meet. Consider this: as of 2025, WHO's VigiBase contained over 40 million Individual Case Safety Reports, yet more than 90% of adverse drug events still go unreported. To protect patient safety and stay ahead of regulatory deadlines, pharmaceutical companies need to embrace intelligent automation.
Each AI tool brings distinct strengths to the table. Deep Intelligent Pharma leverages NLP and machine learning to swiftly extract adverse event data, enabling early detection. Oracle Argus Safety is the top choice for large-scale operations, offering scalable workflows and built-in robotic process automation. Veeva Vault Safety provides cloud-native flexibility with user-friendly interfaces, making it perfect for biotechs needing quick deployment. ArisGlobal LifeSphere uses NavaX AI to cut false positives by 40–50% and speeds up evaluations by 80%. Meanwhile, IQVIA Vigilance Detect excels in multilingual social media monitoring, filtering out 66% of irrelevant content to pinpoint genuine patient concerns.
When selecting an AI tool, focus on your organization’s unique requirements - whether that’s handling high case volumes, leveraging cloud capabilities, reducing noise in data, or ensuring real-time monitoring. Key factors to evaluate include AI explainability, seamless integration with existing systems, and compliance with standards like ICH E2B(R3).
Human oversight remains critical to ensure AI outputs meet quality and compliance standards. As Robert Ball, MPH, ScM, MD from the FDA notes:
"The trustworthiness of the AI algorithm is the main determinant of its acceptance by human experts."
FAQs
How do AI tools enhance the detection of adverse drug reactions compared to traditional methods?
AI tools are transforming the way adverse drug reactions are detected. They boost accuracy, cut down on false positives, and identify potential safety issues much earlier than traditional manual methods. By using advanced algorithms to sift through massive datasets, AI can uncover patterns and connections that might otherwise go unnoticed.
Here’s how AI stacks up against manual methods: it can improve sensitivity by 15–30%, reduce false positives by 20–40%, and spot safety signals 2–6 months earlier. This proactive capability allows for quicker responses to potential risks, enhancing patient safety and helping meet regulatory requirements more effectively.
How do AI tools improve compliance in drug safety monitoring?
AI tools are transforming drug safety monitoring by making the detection of adverse drug reactions (ADRs) faster and more precise. They simplify complex tasks like signal detection, literature analysis, and case management, helping pharmaceutical companies and regulatory bodies adhere to rigorous safety standards set by organizations such as the FDA and EMA.
By automating repetitive processes and enhancing data accuracy, AI reduces the chances of non-compliance linked to human mistakes or delays. These tools also enable real-time monitoring and proactive risk management, ensuring companies can keep up with changing regulations while prioritizing patient safety throughout a drug's lifecycle.
What is the best AI tool for small biotech companies looking for quick and easy deployment?
Lifebit R.E.A.L. stands out as a smart option for small biotech companies thanks to its scalable and automated platform designed for real-time tracking of adverse drug reactions. By simplifying data collection, analysis, and regulatory compliance, it allows companies to set up drug safety monitoring quickly and efficiently.
The platform’s intuitive design makes it easy to implement, enabling smaller firms to save both time and resources while ensuring they uphold strict safety and compliance standards.